Research problem and justification
Rubber plantations are expanding rapidly in Southeast Asia especially in areas where the crop was not historically found. Last decades, more than 1,000,000 ha have been converted in non-traditional rubber growing areas of China, Laos, Thailand, Vietnam, Cambodia, and Myanmar. Almost 97% of the world’s natural rubber supply coming from Asia and nowadays, entrepreneurs in this region tend to invest the crop to other part of the country where it is not traditional rubber plantation area (Fox J. and Castella J.C. 2010).
Thailand is the second largest area of rubber plantation in the world following Indonesia and is the world’s largest producer of natural rubber and also the world leader in rubber wood production and export (LDD 2005a).Since 1989, rubber plantation has gradually shifted from the south to the northeastern of the country and with the action of governmental launch the one million rai (» 160,000 ha) in 2004, it also increased new rubber planting in the north and northeast of the country (Chantuma A, et al. 2012).In eastern region, like other parts of the country has encountered an expansion of rubber plantation. In Chachoengsao Province, our study area, four districts (Plaeng Yao, Phanom Sarakham, Sanam Chai Khet and Tha Takiep) are covered by rubber plantation occupying an area of 126,224 rai (»20,195 ha) in 2011 (Rubber Research Institute of Thailand, 2012) and this might be increased more in the future due to the country tendency in rubber plantation expansion.
Although, rubber plantation is an important commercial crop in Thailand and Southeast Asia, it creates employment and income generation activities for local people, as well as they contribute to community development and to government revenues. However, according to the increasing of the workers demand, hiring the workers from other areas, including Thai or non-Thai workers is considered necessary, population movement contribute toward the impact of expanding of rubber plantation should be monitored intensively. In term of environmental effect, unrestricted expansion of rubber plantation could lead to devastating natural forest. Intensive rubber plantation cannot be comparable to natural forest in term of biodiversity. Similarly, land and other natural resources will be lost because of high demand for land large area for rubber plantation. Growing rubber plantation in the new area was also suggested to be a feasible way to avoid serious outbreak of epidemic diseases that presented in traditional area (Chantuma A, et al.2012). Ecosystem change has influence on the pattern of vector-borne diseases such as chikungunya and malaria (Pattanayak S.K. et al,). Transformation from land to rubber plantation also results in local malaria reemerged and established of malaria transmission in high levels (Pattanayak S.K. et al, Rosenberg et al.1990). Aedes albopictus and Anopheles dirus, main vectors of chikungunya and malaria respectively, are reported to be presented in rubber plantation (Thavara U.et al, 2009, Rochana W.et al, 2010, Sinhasivanon P. et al, 1999, Sumodan P.K., 2003). Our recently unpublished scientific findings regarding vector-borne diseases in rubber plantation in eastern Thailand: 1) demonstrate the co-infection of chikungunya virus (CHIKV) and dengue virus (DENV) in humans and mosquito vectors, 2) indicate the possibility that biting mosquito species other than Aedes may play a role as vectors of dengue and chikungunya, and 3) suggest the possible route of transmission cycle of both diseases among humans and domestic animals living in the same households surrounded by multiple potential vector species (Kittayapong et al., unpublished data).When rubber plantation is introduced within the area, these mosquito species will be also established. Transmission of vector-borne diseases such as chikungunya and malaria within Chachoengao Province might be in question.
This study will apply an ecohealth trans-disciplinary approach which aims to determine whether the expansion of rubber plantation in eastern Thailand increases the risk for vector-borne diseases using chikungunya and malaria as a proxy disease. The relationship between ecological, biological and sociological determinants will also be explore and finally behavior and livelihood of rubber workers related to emerging of vector-borne diseases will be investigated in order to integrate appropriate measures to prevention/control vector-borne diseases presented within the study area.
Research questions
- Why and how the expansion of rubber plantation in eastern Thailand increases the risk for vector-borne diseases?
- Are there innovative solutions and best practices in the occupation associated with rubber plantation that could reduce disease risk?
- Could behavior and livelihood of workers associated with rubber plantation be changed to reduce vulnerability to vector-borne diseases?
- To determine whether the expansion of rubber plantation in eastern Thailand increases the risk for vector-borne diseases using chikungunya and malaria as a proxy disease
- To investigate whether there are solutions and best practice in rubber plantation that could reduce the risk for vector-borne diseases
- To investigate whether behavior and livelihood of rubber workers could be changed to reduce vulnerability to vector-borne diseases
- Ecohealth trans-disciplinary approach to investigate and synthesize the baseline knowledge related to risk of vector-borne diseases in rubber plantations
- Ecohealth trans-disciplinary approach to implement best practice among communities associated with rubber plantations in order to reduce risk of vector-borne diseases
Research objectives
- To determine whether the expansion of rubber plantation in eastern Thailand increases the risk for vector-borne diseases using chikungunya and malaria as a proxy disease
- To investigate whether there are solutions and best practice in rubber plantation that could reduce the risk for vector-borne diseases
- To investigate whether behavior and livelihood of rubber workers could be changed to reduce vulnerability to vector-borne diseases
- Ecohealth trans-disciplinary approach to investigate and synthesize the baseline knowledge related to risk of vector-borne diseases in rubber plantations
- Ecohealth trans-disciplinary approach to implement best practice among communities associated with rubber plantations in order to reduce risk of vector-borne diseases
Research entry points
- Ecohealth trans-disciplinary approach to investigate and synthesize the baseline knowledge related to risk of vector-borne diseases in rubber plantations
- Ecohealth trans-disciplinary approach to implement best practice among communities associated with rubber plantations in order to reduce risk of vector-borne diseases
Research Plan
Our proposed research will employ an eco-bio-social approach to understanding the situation according to the impact of expansion of rubber plantation and increase risk of vector-borne diseases.
Research Phase
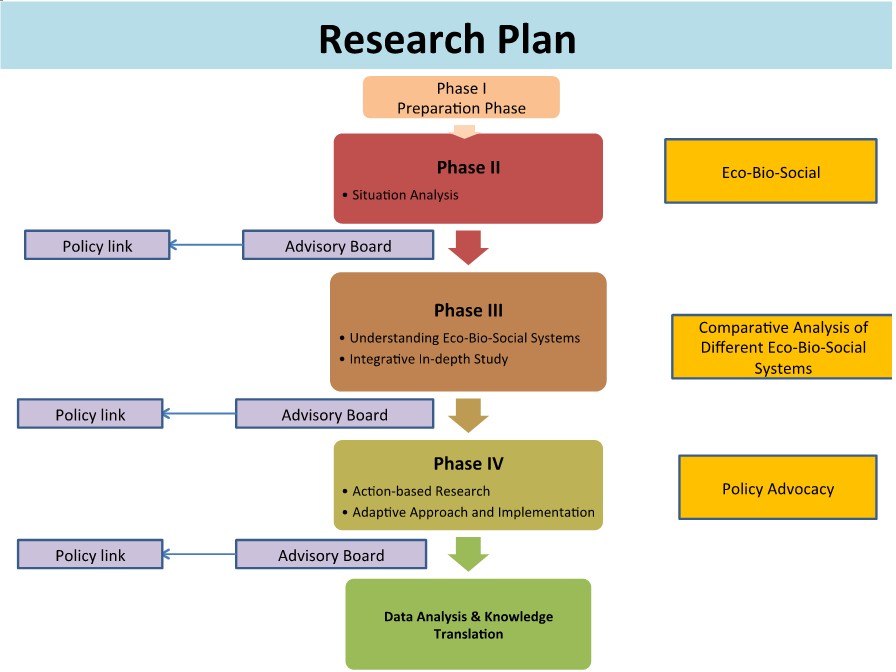
First Phase: Situation analysis
In the first phase, the cross-sectional study will be applied for collecting baseline information including ecological, biological and sociological determinants.
Second Phase: Action-based research
In this phase action-based research co-operated with keys stakeholderswill be performed in selected community/area according to the situation analyzed from first phase.
Third Phase: Knowledge translation/Policy link
Figure 1. Research plan from Phase I to Phase IV
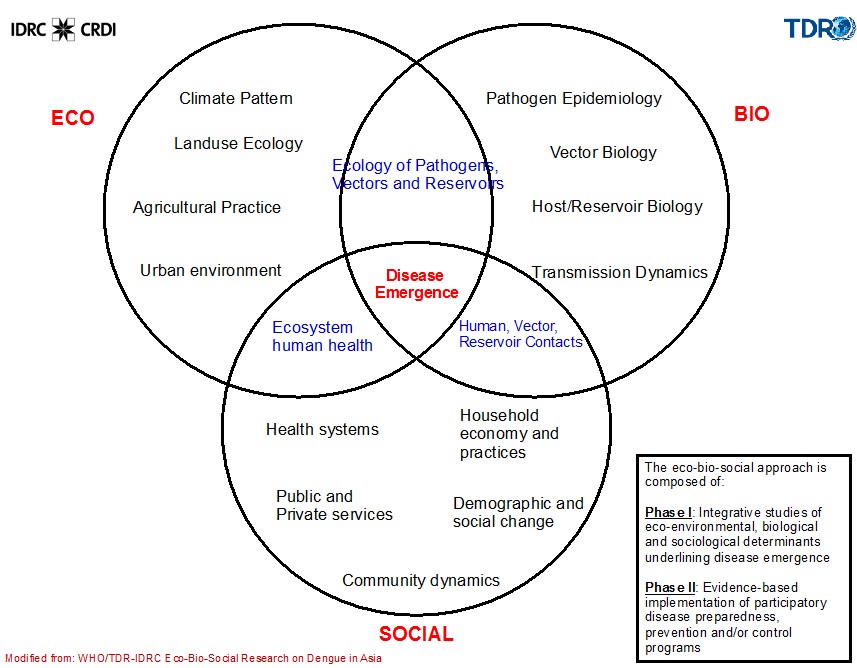
Figure 2. The Eco-Bio-Social Conceptual Framework
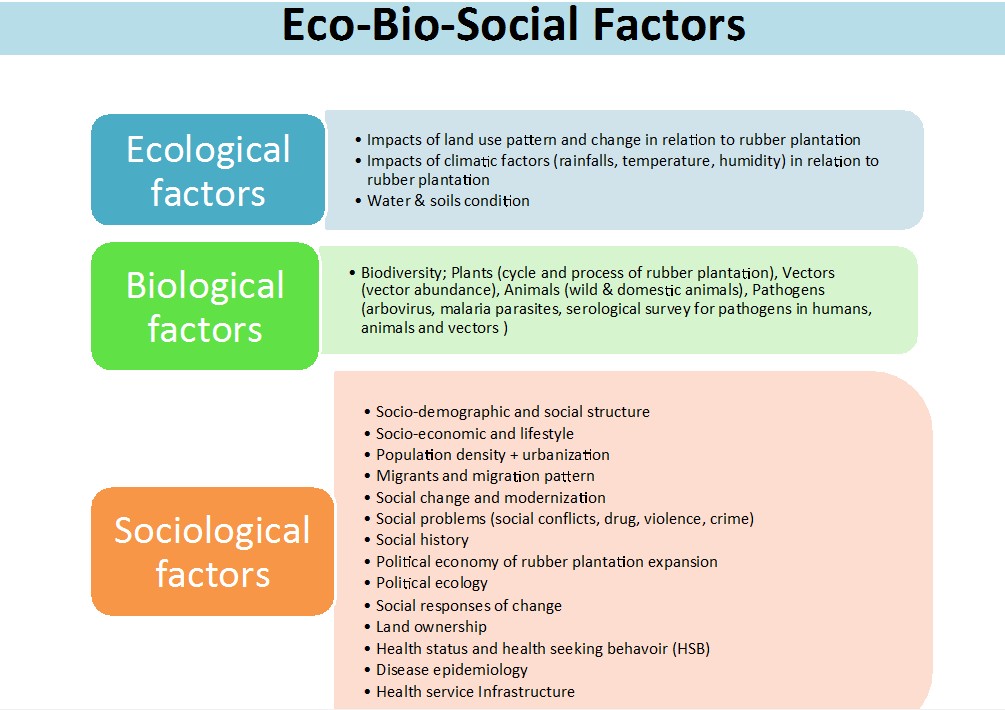
Figure 3. Eco-Bio-Social factors in relation to rubber plantation expansion.
Materials and Methods
Sampling and sample size
Multistage sampling designs will be used to obtain a representative sample from large to small scale according to area under local administration, district to community. We will create a map of study site using Google Earth software (Google Inc., Mountain View, CA, United States of America) and place a grid on it with 200 squares. Then we will number the squares and use simple random number to select the areas for cross sectional study.The sample size reflects a result would give 95% confidence that the true prevalence was <5%.
Ecological factors
Meteorological data: Daily data of rainfall (mm), temperature (°C) and relative humidity (%) registered for the period 2001-2011, will be used in this study. The data will be collected from the Thai Meteorological Department (TMD), Ministry of Information and Communication Technology. Total precipitation data will be collected from 9 rain stations of Chachoengsao. Then, the rainfall data in each district will be estimated from all rain stations using the Inverse Distance Weighted (IDW) interpolation technique. While, minimum, maximum and average temperatures will be collected from only one weather station in the central of Chachoengsao (Kurusattra S.2010).
Land use:Based on land use data in Arcview shapefile that will be collected from the Bureau of Policy and Land Use Plan, Land Development Department (LDD), Ministry of Agriculture, rubber plantation in Chachoengsao Province will be elucidated. Analysis of rubber expansion, land use settings and characteristic of environmental aspects in each district will be studied by using the Geographic Information System (GIS) and remote sensing technology in Arcview software (ESRI, Redlands, CA).
Soil condition and contaminants:The quality of soil is rather dynamic and can affect the sustainability and productivity of land use (cite). It is controlled by chemical, physical, and biological components of a soil and their interactions (Kinyangi J., 2007, Papendick and Parr, 1992).
Biological: Because soil quality is strongly influenced by microbiological mediated processes (nutrient cycling, nutrient capacity, aggregate stability), it is important to identify biological indicators of soil quality (Doran and Parkin, 1994; Abawi and Widmer, 2000). Biological indicators of soil quality that are commonly measured include soil organic matter, respiration, microbial biomass (total bacteria and fungi,) and mineralizable nitrogen.
Chemical:Results of chemical tests are soil quality indicators which provide information on the capacity of soil to supply mineral nutrients, which is dependent on the soil pH. Soil pH is an estimate of the activity of hydrogen ions in the soil solution. It is also an indicator of plant available nutrients. High activity is not desirable and the soil may require liming with base cations Ca or Mg in order to bring the solution back to neutral. Electrical conductivity, sodium adsorption ratio, organic carbon and nitrogen, caution exchange capacity, extractable bases, and also particulate organic matter are also mention by Lewandowski A (1999) to be commonly important indicators of soil quality.
Physical:Soil physical properties are estimated from the soil’s texture, bulk density (a measure of compaction), porosity, water-holding capacity (Hillel, 1982). The suitability of soil for sustaining plant growth and biological activity is a function of its physical properties (porosity, water holding capacity, structure, and tilth).
Water condition and contaminants Surfaces water will be collected from rivers and stream at study area to examine chemical, physical and microbiological quality by using standard procedure of APHA (1989). Water samples for microbiological analysis should immediately be placed in a lightproof insulated box containing melting ice or ice packs while collection and during the transportation. The delay time between collection and analysis should not exceed 6 hours and 24 hours is considered the absolute maximum. If water samples contain trace of chlorine, it should be inactivated in order to preserve microbes living in the samples. While water samples for physicochemical analysis should be kept in glass or polyethylene bottles at low temperature in the dark. Residual chlorine, pH and turbidity should be test immediately after sampling as they will change during storage and transport. The water samples will be subjected to filtration prior to chemical analysis. The determination of TDS (Total Dissolved Solid) will be done by a gravimetric process, while the total hardness will be carried out by EDTA complexometric titration method. For analysis of DO and BOD, it will be performed by using the Winkler’s method, while analysis of nitrate will be done by colorimetric procedure. Fecal coliform population will be analyzed by MPN/100ml method, by growing on M-FC medium at temperature 44.5ºC ± 1 ºC and counted after 48 hours.
Biological factors
Biodiversity of animals and plants
The data about diversity of wild animal and plant will be obtained from Office of Conservation Area 2 (Sriracha), National Park, Wildlife and Plant Conservation Department which takes responsibility for the province of Chonburi, Rayong, Chantaburi , Chachoengsao, and Trat. This data describes about wild animal and plant species that has been found and recorded in study area. While the information of domestic animal, it will be obtained from Chachoengsao provincial livestock office.
Vector abundance
Sampling of mosquito larvae and pupae:Collection of immature will be conducted according to the protocol developed in other similar studies in Thailand and other countries (World Health Organization, Strickman and Kittayapong (2002, 2003), TDR-Pupae project). Briefly, water storage containers (jars and cement bath basins) and drinking water containers will be examined with a flashlight and the present of immature recorded. All visible larvae and pupae will be hand-collected or by dipping. The immature in other containers (tires, flower pots, discarded containers, ant trap, etc) will be sampled by dipping with fine-meshed fishnet (diameter of 24 cm) or by pouring out the water in a bottle for the smallest containers. To collect the larvae in containers, the dipping started from top of the containers and continues to the bottom in a swirling motion covering all edges of the container. The number of pupae in each container will be recorded. Pupae will be reared to the adult stage in a small 50 ml tube for further specie identification.
Sampling of adult mosquito in the field:Mosquitoes will be collected by using a variety of trap including BG trap, CDC light trap, and portable vacuum aspirators in order to increase species diversity of mosquito. BG traps will be set up in each sampling site in the morning and mosquitoes were collected in evening of the same day and the next morning. While light trap will be set up in the evening and mosquito will be collected in the next morning. For portable vacuum aspirators, mosquitoes will be mainly collected by both indoor and outdoor of house located nearby the rubber plantation. All sampling sites will be located by using Geographic Positioning System (GPS) not only to show spatial distribution of sampling site, but also demonstrate spatial distribution of mosquito in study site. Collected mosquitoes will be kept in 1.5 ml microcentrifuge tube, whereas live mosquitoes. All mosquito samples will be stored in refrigerator and upon arrival to the laboratory at the Center of Excellent for Vectors and Vectors-Borne Diseases (CVVD), Faculty of Science, Mahidol University, samples will be stored at -80ºC until used.
Mosquito Identification:After arrival to the laboratory, all mosquito samples will be sorted, performed on cooling plate, by sex, blood meal status and species using illustrated keys to the mosquitoes of Thailand (Rattanathikul et al, 2005). Mosquito samples in different species and sex will be placed individually in separate tube. Later, dissection of mosquitoes will be performed to separate the head, thorax and abdomen. Then mosquito samples will be stored at -80ºC until used for RNA and DNA extraction, blood meal analysis and screening of chikungunya and malaria.
Identification of mosquito blood meal pattern by an Enzyme –Linked Immunosorbent assay technique (ELISA):An Enzyme –Linked Immunosorbent assay technique (ELISA) will be performed to examine blood meal source of engorged blood female mosquitoes according to the method previously described by Thapar et al, 1998. Blood meals will be identified by ELISA technique using anti-host (IgG) conjugates (Kirkegaard and Perry, Gaithersburg, MD) against human, domestic animal such as dog, cow and monkey. The 96 well polyethylene plate will be coated with 50 µl of antibody diluted in carbonate buffer and incubate overnight at 4ºC. Coated plate will be washed 3 times with PBS- 0.05 % Tween20 using washing machine and then the plate will be blocked with 50 µl of 0.5 % casein in PBS pH 7.4, followed by washing three times with PBS- 0.05 %Tween20. The abdomen of each mosquito will be ground in 100 µl of phosphate-buffered saline (PBS) and then diluted to 1: 200 using 0.5 % casein in PBS pH 7.4 - Tween 20 0.05% as diluent. A volume of 50 µl of positive, negative control and test sample will be added to duplicated wells and incubated for 1 hour, followed by washing three times. Then, 50µl of antibody-enzyme conjugated (HRP-antibody enzyme conjugated in 0.5% casein - PBS – Tween 20 0.05%) will be added in well and incubated 1 hour at 37ºC. Plate will be washed 3 times and 50 µl of substrate solution composed of citrate phosphate buffer, O-Phenyldiamine Dihydrochloride tablet (OPD) and 3% hydrogen peroxidase will be added on each well. Then plate will be kept in the dark room for 30 minutes. Reaction will be stopped by adding 50 µl of 4M H2SO4 in all wells. Absorbance value will be read by ELISA plate reader at wavelength of 490 nm. Test specimen will be considered to be positive when absorbance value will be greater than the mean cut off plus 2 two standard deviations of the negative controls.
Serological survey
Blood specimens will be taken from rubber workers and also from domestic animal living near by the rubber plantation. Clotted blood samples will be collected and centrifuged at 3,000 rpm for 5 minutes. All samples will be kept at 4 ºc in an ice chest for less than two hours until transportation to the laboratory and they will be then kept at -80 ºc until use. Chikungunya and malaria infection will be detected by reverse transcriptase polymerase chain reaction (RT- PCR) (Edwards et al, 2007). and polymerase chain reaction (PCR)( Snounou et al, 1993) respectively.
Pathogen detection
Detection of chikungunya virus by RT-PCR
RNA extractionThe genomic viral RNA will be extracted from 100 µl of human or animal serum or from mosquitoes. Individual mosquitoes are ground in 100 µl of PBS at 3000 rpm for 3-5 minutes using Tissue lyser II, with aids of 3 mm bead. Grinding mosquito solution or serum sample are homogenized with 750 µl of Easy-Red TM solution, followed by vortex for 30 seconds at maximum speed and stores the tube at room temperature for 5 min. Then, 200 µl of chloroform are added, followed by vortex for 30 seconds and stores the tube at room temperature for 5 min. The tube is centrifuged at 13,000 rpm at 4°C for 15 minutes. The upper phase of the tube is transferred to a new microcentrifuge tube and 500 µl of Isopropanol are added to the tube, mixed by inverting the tube 4-5 times. After incubated at room temperature for 10 min, the tube is centrifuged at 13,000 rpm for 10 minutes at 4°C. The supernatant are removed carefully and 1 ml of 75% ethanol is added to the tube to remove impurities from RNA pellet. After centrifuge at 13,000 rpm for 5 minutes at 4°C, the supernatant are discarded without disturbing the pellet and the remaining RNA pellet are dried for 15 minutes. Later, RNA are dissolved by adding 20 µl of DEPC water and stored at -20°C until used.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)A Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) is performed to detection and identification of chikungunya, as previously described (Edwards et al, 2007). Briefly, 2.5 µl of RNA extract suspension is mixed with master mix composing of 12.5 µl of 2x One step buffer, 0.5 µl of forward primer CHIK1 (TATCCTGACC ACCCAACGCTCC), reverse primer CHIK2 (ACATGCACATCCCACCTGCC) and RNase inhibitor, 1 µl of enzyme mix and 7.5 µl of RNase-free water. Virus cDNA are synthesized and amplified by PCR machine carried out 40 cycles at 50ºC 30 min, followed by 94°C for 2 min, 94°C for 15 sec, 55°C for 30 sec, 68°C for 20 sec, 68°C for 5 min and hold on at 20°C. After cycling, 5 µl of PCR product are loaded in a 2% agarose gel in TAE buffer strained with ethidium bromide, followed by visualized under UV light inside GelDoc machine.
Detection of malaria by Polymerase Chain Reaction (PCR)
Extraction of malaria parasite DNA:Field wild caught mosquitoes or blood samples are homogenized in 50 µl of blocking buffer and Nondiet P-40. Two hundred µl of the Instragen (BioRad) are added to 20 µl of this homogenate and then incubated at 56°C for 30 minutes. After vortex at high speed for 10 seconds, this homogenate are heated at 100°C for 8 minutes and then vortex at high speed. It is centrifuged at 12000 rpm for 3 minutes at room temperature. The supernatant is transferred to new tube and stored at -20°C until used in PCR analysis.
Polymerase Chain Reaction (PCR)To detect the presence of Plasmodium in mosquito or in blood samples, Polymerase Chain Reaction (PCR) is performed by using universal primers, including the Nest 1 primer sequences: rPlu.1 (TCA AAG AAT AAG CCA TGC AAC TGA CCT GTT GTT GCC TTG AAC TCC), rPlu5. (CGT GTT GTT GCC TTA AAC TCC) (Snounou et al, 1993) and the Nest 2 primer sequences: rPlu3. (TTT TTA TAA GGA TAA CTA CGG AAA AGC TGT) and rPlu4. (TAC CCG TCA TAG CCA TGT TAG GCC AAT ACCA) (Singh et al, 1999). Briefly, 3 µl of DNA template is mixed with 20 µl of master mix composing of 9.6 µl of H2O, 2 µl of 10x reaction buffer, 2.4 µl of 25nM MgCl2, 1 µl of deoxynucleotide triphosphate (dNTPs) to final concentration of 200 µM and 10 pmol of each primer. After denatured at 100°C for 5 minutes and then chilled on ice, the mixture is added with Taq polymerase (2.5 units Fermentas). PCR amplification is carried out 35 cycles with denaturation at 94°C for 1 min, annealing at 56°C for 1minute and extension at 72°C for 1minute. For Nest 2 PCR, 3 µl of first PCR product was mixed with similar master mix as Nest 1 and amplified in PCR machine with the same condition, except that it is annealed at 62°C.After cycling, 10 µl of PCR products are loaded in a 2% agarose gel in TAE buffer strained with ethidium bromide, followed by visualized under UV light inside Gel-Doc.Sample that is positive for Plasmodium will be further analyzed with specific primers, which is rFAL1 (5’-TTA AAC TGG TTT GGG AAA ACC AAA TAT ATT-3’) and rFAL2 (5’-ACA CAA TGA ACT CAA TCA TGA CTA CCC GTC-3’) for P. falciparum, rVIV1 (5’-CGC TTC TAG CTT AAT CCA CAT ACC TGA TAC-3’) and rVIV2 (5’-ACT TCC AAC CCG AAG CAA AGA AAG TCC TTA-3’) for P. vivax, rMAL1(5’- ATA ACA TAG TTG TAC GTT AAG AAT AAC CGC-3’) rMAL2 (5’-AAA ATT CCC ATG CAT AAA AAA TTA TAC AAA) for P. malariae and rOVA1(5’- ATC TCT TTT GCT ATT TTT TAG TAT TGG AGA) and rOVAL2 (5’-GGA AAA GGA CAC ATT AAT TGT ATC CTA GTG) for P. ovale. Three microliters of Nest1 is added to PCR mixture identical to those Nest1 previously mentioned and the PCR amplification is carried out for 45 cycles. The annealing temperature for each species is slightly different: at 58°C for P. falciparum and 60°C for P. vivax. After cycling, 10 µl of PCR products are loaded in a 2% agarose gel as previously described.
Sequencing analysis
Nested PCR product considered to be positive will be purified by using NucleoSpin ®Extract II column and quantified. Sequencing of amplified fragment will be performed by using BigDye Terminator v3.1 Cycle Sequencing Kits (ABI-PRISM 3100/3130 Genetic Analyzer, Applied Biosystems). The obtained sequences will be compared with sequences available in the GenBank library by applying a basic alignment search tool (BLAST). Phylogenetic trees will be reconstructed by the program MEGA 4 (Tamura et al. 2007).
Sociological factors
Epidemiological data: Daily reported cases of Chikungunya and malaria, and other diseases during the past 11 years from 2001- 2011 will be used in this study. The data will be collected from the Center of Disease Control, the Provincial Health Office of Chachoengsao Province. The data will be obtained from R506 report using code number such as 30- Malaria and 84- Chikungunya. The form 506 provided data for each patient's age, gender, address, occupation, race, date of sick and date of define.
Demographic data:Human populations in the selected communities will be addressed at a relevant scale as part of the process of describing the ecosystem and its boundaries. This will include consideration of migrants and migration pattern, as well as cultural and socioeconomic differences that identify distinct human populations as key stakeholder groups. Characteristics such as the degree to which these populations are mobile, lifestyles, daily routines, social history, political ecology, social problems, health status, health seeking behavior, health resources and outreach, will be identified by using data collected from documentary records, key informant interview, focus group discussion, cross sectional survey and or together with observation by the researcher teams.
Socio-demographic and social structure: Information according to socio- demographic and social structure will be collected by household interview following standard questionnaire together with focus group discussion
Socio-economic and lifestyle: Data will be collected by self-interview, observation by well-trained staffs. Standard questionnaire will be developed for collecting the information including the household income, source of major income, the routine schedule of people in community, etc.
Population density and urbanization: The population density and urbanization will be obtained by following the report of Department of Provincial Administration, combined with the data collected from Chachoengasao Office of Public Works and Town and country Planning.
Migrant and migration pattern: Data will be obtained by the report of Department of Provincial Administration,Local Administrative office together with household interview and observation.
Social change and modernization: Data will be collected from Provincial and Local Administrative offices, Local Cultural Learning Center, combined with household interview, focus group discussion and observation.
Social problems (social conflicts, drug, virulence, crime): Data will be obtained by many sources such as Department of Provincial Administration, Chachoengsao Provincial Health Office, Local administrative Office, etc. and data directly collected by interview people in community, focus group discussion and observation.
Social history: Data will be collected from Provincial and Local Administrative offices, Local Cultural Learning Center, combined with household interview, focus group discussion and observation.
Political economy of rubber plantation expansion: Data will be collected from both government and private sector organization that involved in rubber plantation such as Chachoengsao Rubber Research Center, Rubber Plantation Organization, and Office of Rubber Replanting Aid Fund, organizations of rubber farmers and rubber processing operators, together with the focus group discussion of rubber farmers.
Social response of change: Information will be collected from interview according to standard questionnaire, focus group discussion and observation.
Land ownership: Information will be collected by using the record of Chachoengsao Lands office, Phanom Sarakham branch, together with household interview and observation.
Health status and Health Seeking behavior (HSB): Data will be collected from Chachoengsao Provincial Health Office combined with self-interview, focus group and observation.
Health service infrastructure: Data will be collected from Chachoengsao Provincial Health Office.
Data analysis
Descriptive statistics will be used to describe the distribution of vectors, animals and pathogens in the area. Ecological, biological and sociological determinants will be indentified and categorized, logistic regression will be used to test the association between each variables.
Construction of geo-epidemiological map of the study area:The retrospective chikungunya and malaria cases during 2001-2011 will be manipulated in Arcview software by using the digital base map provided by the Royal Thai Survey Department at the scale of 1:50,000. Then, manipulation of the GIS data and production of the epidemiological map of the study area by using GIS technology will be done.
Relationship between climatic factors and number of cases:Daily rainfall data collected from 9 rain stations (Sanam Chai Khet 1, Sanam Chai Khet 2, Amphoe Muang, Ban Pho, Bang Kha, Bang Nam Prieo, Bang Pakong, Ratchasan and Tha Takiap) will be calculated by using the Inverse Distance Weighted (IDW) interpolation technique then it will be grouped to bi-weekly data. For daily temperature (minimum, average, maximum) that will be collected from a weather station in Amphoe Muang, it will be later grouped into bi-weekly. Then, relationship between number of chikungunya, malaria case and climatic factors will be evaluated in bi-weekly basis by using time lag series analysis. The coefficient correlation between number of case and climatic factors will be elucidated by using multivariate regression analysis.
Social and environmental aspects of the study area:The socio-demographic and environmental data will be integrated and stimulated into GIS (Savane) developed by Marc Souris from the Mahidol University team; ArcMap software (ESRI, Red land, CA) to characterize chikungunya and malaria epidemics situation (Souris Pers.Comm., Barbazan et al. 2002, Nitatapatana et al. 2006). Rubber plantation expansion will be calculated in km2 and evolution/pattern of expansion will be characterized. Statistical analysis (regression, ANOVA) will be done using statistical software (SPSS, SAS).
Engagement of multi-stakeholders in the strategy development process
Various stakeholders, i.e., local government, NGOs, investors, migrants, labors, and etc. involving in the expansion of rubber plantation will be identified. Attempt will be made to engage these stakeholders at the earliest stage. Multi-stakeholders and their roles identified from the stakeholder analysis will be emphasized. A few communities of practice (CoP) meetings will be organized to set a stage for community dissemination of evidence-based information and multi-disciplinary approach action to mitigate the risks.
Knowledge translation, communication and policy link
Policy link via reports and recommendations to policy makers: Results gained from these proposed projects will be transferred in the form of reports and recommendations to public health end users (Ministries of Health) in each participating country after the project ends. Community of practice (CoP) workshops organized at the beginning and at the end of the project will ensure engagement of policy makers and/or end users from each participating country.
Knowledge translation and communication via publications: Project findings will be published in peer review journals to inform wider communities on the findings related to emerging and re emerging diseases.
References
v APHA 1989. Standards Methods for the Examination of Water and Wstewater. 17th Edn. Wachington.D.C: APHA, AWWA, WPFC, 2005.
v Authayanraksa N. 2007. Environmental and Techno-Policy Analysis of an Agro Eco-Industrial Network in Chachoengsao Province. Thesis. M.Sc (Environmental Enginering and Management). Asian Institute of Technology. p 1-170.
v Chan YC, Chan KL, Ho BC. Aedes aegypti(L) and Aedes albopictus (Skuse) in Singapore:5. Observations in relationship to dengue haemorrhagic fever. Bull of WHO 971;44:651-8.
v Chantuma A, et al. Rubber New Planting in Thailand: Towards the World Affected on Climate Change. Rubber Thai Journal 2001;1:40-47.
v Edward CJ, Welch SR, Chamberlain J, Hewson R, Tolley H, Cane PA, et al. Molecular diagnosis and analysis of chikungunya virus. J Clinic Virol 2007;39:271-275.
v FAO 2006. Faostat data, 2006.[www-document] http://faostat.fao.org
v Fox J and Castella JC. Expansion of rubber (Hevea brasiliensis) in Mainland Southeast Asia: What are the prospects for small holders? 2010:1-21.
v Kinyangi J. 2007. Soil health and soil quality: A review.1-16.
v Kurusattra S. Identify risk area of dengue heamorrhagic fever using spatial analysis in Chachoengsao Province. Thesis. M.Sc (Industrial Ecology and Environment). Mahidol University.2010:1-168.
v Land Development Department of Thailand. Para-rubber (Thai economic crop) and soil suitable for planting in the east.2005a. [www-document]
v http://www.ldd.go.th/EFiles_project/article/article06.html
v Laura Rantara. Rubber plantation performance in the Norhteast and East of Thailand in relation to environmental condition. M.S. Thesis. Department of Forest Ecology/Viikki Tropical Resources Institute (VITRI), University of Helsinki, Finland. 70 p.
v Lewandowski A, Zumwinkle M and Fish A. Assessing the soil system. A soil quality literature review. Energy and Sustainable Agriculture Program. Minnesota Department of Africulture.1999; 1-65.
v Pattanayak S.K. et al.Deforestation and malaria: Revisiting the human ecology perspective.
v Rattanarithikul R, Harrison BA, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distribution; lists of genera, and species; and a key to the genera. Southeast Asian J Trop Med Public Health 2005; 36 (Suppl 1): 1–80.
v Rochana W, et al. Risk factors for chikungunya fever in Thepha and Chana District, Songkha Province, March-April 2009. Weekly epidemiological surveillance report. 2010; 41: 36-40.
v Rubber Research Institute of Thailand, 2012. World Rubber Statistics.
v Rubber Research Institute of Thailand, 2012(a). Technical report 2010. Department of Agriculture. Minisrty of Agriculture and Cooepratives. p1-121.
v Singh, B., Bobogare, A., Cox-Singh, J., Snounou, G., Abdullah, M. S., & Rahman, H. A. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 1999;60: 687–692.
v Sinhasivanon P. et al. Malaria in tree crop plantations in South-Eastern and Western Provinces of Thailand. SEA J Trop Med Publ Hlth 1999; l30:399-404.
v Snounou, G. (n.d.). Detection and Identification of the Four Malaria Parasite Species Infecting Humans by PCR Amplification. Species Diagnostics Protocols 50: 263–292. New Jersey: Humana Press.
v Strickman, D., & Kittayapong, P. Dengue and its vectors in Thailand: Introduction to the study and seasonal distribution of Aedes larvae. Am J Trop Med Hyg 2002; 67:247–259.
v Sumodan P.K. Potential of Rubber Plantations as Breeding Source for Aedes albopictus in Kerala, India. Dengue Bulletin 2003;27:197-198.
v Tamura, K, Dudley, J, Nei, M, Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 2007; 24:1596–1599.
v Thapar BR, Sharma SN, Dasgupta RK, et al. Blood meal identification by using Microdot ELISA in vector mosquitoes. J Commun Dis 1998;30:283-7.
v Thavara U, et al. Outbreak of chikungunya fever in Thailand and virus detection in field population of vector mosquitoes, Aedes aegypti (L.) and Aedes albopictus Skuse (Diptera: Culicidae). SEA J Trop Med Publ Hlth 2009; 40:5:951-962.
v Vythilingam, I.,Nitiavathy, K.Yi, P., Bakotee, B., Hugo, B., Singh, B., Wirtz, R. A., et al. A highly sensitive, nested polymerase chain reaction based method using simple DNA extraction to detect malaria sporozoites in mosquitos. SEA J Trop Med Publ Hlth 1999; 30: 631–635.
v Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology.Am J Trop Med Hyg2007; 76:450–60.